The production, or breeding, of tritium is one of the major engineering challenges of fusion. The easiest fusion reaction uses deuterium and tritium; it requires the lowest temperature and can return the most energy. The core reaction fuses one deuterium and one tritium, producing one alpha particle, which is to say a helium 4 nucleus, and one neutron. The alpha particle has one fifth of the energy (3.5 MeV) and the neutron takes the remaining four fifths (14.1 MeV).

This introduces a challenge because tritium is not a stable isotope. The half-life is 12 years; medium length on a human timescale, but nothing on geological or astrophysical timescales. This means that there is no natural abundance, it decays too quickly, and therefore any fusion power plant must produce its own tritium in a closed cycle.
There are many reasons that tritium is challenging. It is not a stable isotope, as in, it is radioactive. It is also hydrogen, which makes it “leaky”, able to percolate through containment structures. And it is also chemically identical to hydrogen, as in, it is flammable. This all complicates the plant required to extract, purify, store, and recirculate produced tritium into the machine. For these reasons tritium is difficult, but these things are solvable. There is, however, a deep-seated nuclear physics constraint that makes closing the fuel cycle, producing enough tritium, very challenging for some fusion power plant designs.
Tritium is produced using a reaction between the neutron from the fusion reaction itself and lithium. The output of this reaction is another helium 4 and one tritium. The problem is that we have used exactly one tritium to make exactly one neutron, to make exactly one tritium. To make this work we would need to capture every single neutron. Every single one must react with lithium and produce tritium. And we’d have to usefully extract every single atom of tritium produced. Neither of these things are possible.

Fortunately, this is still a simplification, one commonly used and it is normally where the story stops. In fact, there are two stable isotopes of lithium, lithium 6 and lithium 7, and the reaction with the neutron is different between the two. With lithium 7, we get a neutron back out again. This neutron can then go on to interact with another lithium and produce a second tritium. For one neutron in, we can get more than one tritium out.

But again, this is STILL a simplification! There are many things that can happen when a neutron collides with a lithium nucleus. The TENDL-2019 database of neutron cross sections has 88 entries for lithium. All of these entries describe a different possible outcome from the event. Only two produce tritium, and only five produce further neutrons, of which the reaction with lithium 7 mentioned above is the most likely. The rest are mainly different types of “scattering”, different ways that the neutron just bounces off the lithium nucleus.
Each of these processes has a different likelihood of occurring, and that likelihood or “cross-section”, depends on the energy of the neutron coming in. The lithium 7 reaction that gives both a neutron and a tritium works well at the “birth” energy of the neutron, 14.1 MeV, the amount of energy it has just after fusing. However, the lithium 7 reaction is endothermic, meaning that the energy of the output neutron is less than the input neutron, which means that the probability of another lithium 7 reaction is lower. You can’t have an endless process going round and round producing more and more tritium each time. And anyway, scattering is overall the more likely outcome, which also lowers the neutrons energy and makes the lithium 7 reaction less likely. This reaction falls off a cliff as the neutron energy goes down, and that is the direction that nature is taking it.
Eventually, at low energies, when we have “thermal neutrons”, the tritium producing reaction with lithium 6 becomes the most likely process, so thermal neutrons produce tritium very well, but this reaction consumes the neutron.
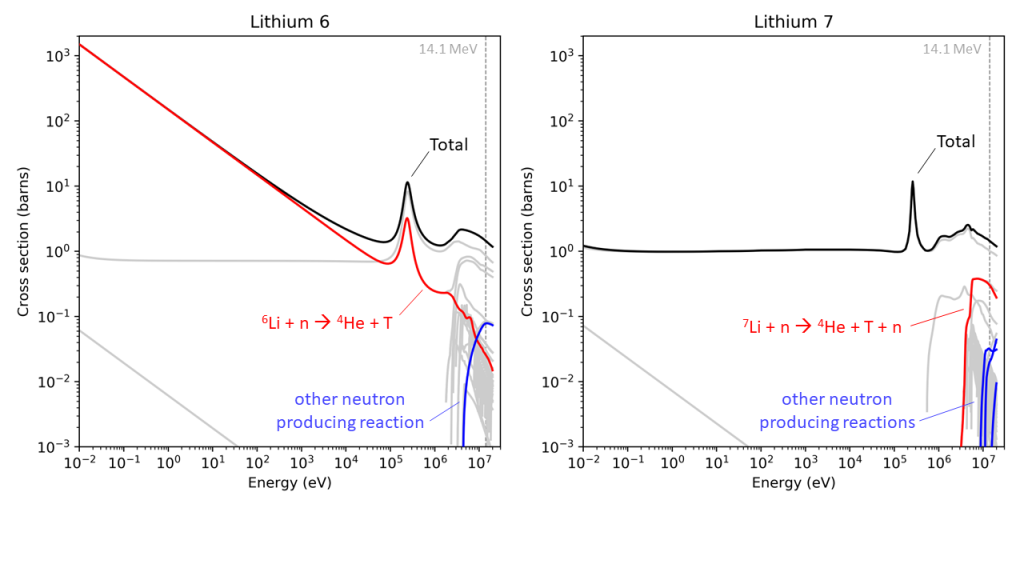
So, thinking of the process as one reaction with lithium 6 and another with lithium 7 is a pretty good approximation. But the outcome of all the detail about energies, cross-sections, and scattering is that there is a limit to the maximum number of tritium atoms one can produce per neutron. In the field this is called the “tritium breeding ratio” and the nuclear physics says it can never be higher than 2. It can be changed by changing the ratio of the two isotopes of lithium, a common proposal for fusion power plant designs struggling to close the loop and produce enough tritium, a very challenging proposal as there is no supply chain for enriched lithium, but it can’t get above this limit of 2.
There are also other ways to introduce neutron multiplication. There are spallation-like reactions with both beryllium and lead that are often discussed. Beryllium is often introduced through the use of FLiBe, a blend of the salts lithium fluoride and beryllium fluoride, acting as a molten salt coolant. And lead is often introduced through the use of lead-lithium eutectic mixture as the coolant. Both of these options lead to the liberation of a neutron from the target nucleus, i.e. the beryllium or the lead, and produce a lighter isotope of the same as the other product. Regardless of all the options, and even combining these with the option of enrichment of the lithium, it is not possible to produce more than two tritium atoms per neutron.
And these additions that can improve the tritium production do not come for free. Beryllium is highly toxic, as is lead. FLiBe introduces fluorine chemistry, and yes, tritiated hydrofluoric acid will be produced. Lead also has a highish atomic number and the range of different isotopes produced rather challenges the “no radioactive waste” statement, polonium being one of the eventual products.
Some suggest using fissionable material, e.g. uranium. The neutron multiplication possible by this route is higher, and the tritium breeding ratio can be greater than two. But why bother? My view is that if you are going to have uranium in your plant, you may as well build a fission plant. You will have all the same difficulties to deal with and fission already works.
This fundamental limit on the amount of tritium that can be produced per neutron is the deep-seated reason that closing the fuel cycle is a challenge. If you cannot capture more than 50% of the neutrons for tritium production, you fundamentally cannot close the loop and be tritium self-sufficient. Space used for other things eats into the total amount of neutrons being captured. In magnetic fusion, neutral beam heaters, RF heaters, the magnets themselves, the central column in a tokamak, the first wall and a host of other things all eat into the space available. In laser inertial fusion, the entrance ports for the many laser beams have the same impact. And in designs that are cylindrical in nature, the ends of the cylinder cannot typically be used for tritium production.
Fortunately for First Light, we can capture 99% of the neutrons in the flowing lithium coolant inside the reaction vessel. We can achieve a tritium breeding ratio of up to ~1.5 simply with normal lithium, no additives, and no enrichment. Any when I say fortunately, what I of course mean is, we deliberately designed it that way.
Learn more here…
https://firstlightfusion.com/technology/power-plant

General Fusion’s approach should also capture almost all the neutrons produced from DT fusion, with their steampunk piston driven compression of a spinning metal fluid. Yet this article from 2017 says their spinning metal is liquid lead-lithium, so presumably they think a neutron breeding additive is needed. Do you understand why they think they need to add this but you do not? https://generalfusion.com/post/magnetized-target-fusion-general-fusion-sofe-2017/
LikeLike
It’s a good question and I don’t understand why they propose to use lead-lithium. There is something that would be quite different, which is the compression of their plasma. Lead-lithium is denser and would compress the plasma more. I do know that they are planning pure lithium for their next machine, but are still talking about lead-lithium for the plant. I don’t really know, in short
LikeLike
Chris, after thinking about your question I was surprised to learn that, if the goal is to find blanket materials with high tritium breeding ratios (TBRs), pure lithium is not worth considering. Its TBR barely exceeds one. Lead-lithium is higher but still under two. To get above two without contaminating the blanket with “dirty” radioactive materials, a mixture of lithium-6 and beryllium looks promising. In order to get more accurate numerical values for the TBRs, an AI bot suggested running multi-batch simulations of the blanket material using a Monte Carlo N-Particle radiation transport code (MCNPX). If someone has access to that software, a better understanding of TBRs can be obtained if they post the result of running simulations on the following three blanket materials: (5% 6Li, 95% 7Li); (5% 6Li, 95% 9Be); (5% 6Li, 95% 206Pb). Thanks 🙂
The way I see it, a blanket’s TBR correlates directly with the average number of neutrons it can spawn from a single 14.1 MeV fusion neutron. With that in mind, the primary function of the blanket is to multiply neutrons. Converting those neutrons into tritium is a secondary function which should not require much 6Li. Lithium-6 is not a neutron multiplier – extra (spallation) neutrons are not produced when neutrons collide with 6Li nuclei. When a neutron encounters a 6Li nucleus, it is either captured or rebounds elastically. If it is captured (temporarily absorbed into the nucleus), the nucleus quickly splits into two pieces, tritium and helium. The overall effect of 6Li is to produce exactly one tritium per fusion-generated neutron. Breeding tritium does not requires a significant amount of 6Li compared to the other components of the blanket. This is because the thermal neutron cross section of 6Li increases to a level several thousand times higher than that of other proposed blanket materials, and, in addition, the n+6Li->4He+3H reaction is highly exothermic (+4.78 MeV).
The following nuclear reactions apply to blanket materials that were discussed on Nick’s blog, 6Li, 7Li, 206Pb, and 9Be. The energies were calculated using E=mcc with the atomic mass units listed at https://pubchem.ncbi.nlm.nih.gov/periodic-table/.
u = 931.4936148 MeV
n + 6Li -> 3H + 4He [+0.00513526785u = +4.78 MeV]
n + 7Li -> 3H + 4He + n [-0.00264910119u = -2.47 MeV]
n + 7Li -> 7Li + n (elastic) rebound v = 6/8, E = 0.563
n + 9Be -> 4He + 4He + n + n (n, 2n) [-0.00168836214u = -1.57 MeV]
n + 9Be -> 8Be + n + n (n, 2n) [-0.00178695588u = -1.66 MeV]
n + 9Be -> 10Be (?) [+0.00731328588u = +6.81 MeV]
n + 9Be -> 9Be + n (elastic) rebound v = 8/10, E = 0.64
n + 206Pb -> 205Pb + n + n (n, 2n) [-0.00868138788u = -8.09 MeV]
n + 206Pb -> 207Pb (?) [+0.00723330488u = +6.74 MeV]
n + 206Pb -> 206Pb + n (elastic) rebound v = 205/207, E = 0.981
————————————————————————-
1n 1.00866491588
3H 3.01604928132 ± 0.00000000008 12.32 y ± 0.02 β-=100%
4He 4.00260325413 ± 0.00000000016 Stable IS=99.9998±0.2%
6Li 6.01512288742 ± 0.00000000155 Stable IS=4.85±17.1%
7Li 7.01600343426 ± 0.0000000045 Stable IS=95.15±17.1%
8Li 8.022486244 ± 0.00000005 838.7 ms ± 0.3 β-=100%; β-α=100%
8Be 8.005305102 ± 0.000000037 81.9 as ± 3.7 α=100%
9Be 9.012183062 ± 0.000000082 Stable IS=100%
10Be 10.013534692 ± 0.000000086 1.387 My ± 0.012 β-=100%
205Pb 204.974481682 ± 0.000001228 17.0 My ± 0.9 ε=100%
206Pb 205.974465210 ± 0.000001228 Stable ± >2.5Zy IS=24.1±3%; α ?
207Pb 206.975896821 ± 0.000001231 Stable ± >1.9Zy IS=22.1±5%; α ?
To answer the question, why does General Fusion plan to include Pb in its blanket, requires an analysis of TBRs, so lets compare the neutron multiplication ability of the candidates: 7Li, 206Pb, and 9Be. Three things need to be considered, (1) the minimum neutron activation energy needed to overcome and trigger an endothermic n,2n reaction, (2) the relative cross section of the n,2n reaction compared to the total (mostly elastic scattering), and (3) the proportion of energy lost per elastic collision (this determines how many collisions can take place before a neutron’s energy drops below the minimum cutoff activation energy). More details, about those three things, follow.
First, the minimum n,2n activation energies, as estimated from Evaluated Nuclear Data File (ENDF) plots, generated using a servlet at https://www.nndc.bnl.gov/endf/, are as follows:
7Li (n,2n) minimum energy ~ 8.30 MeV,
9Be (n,2n) minimum energy ~ 1.75 MeV,
206Pb (n,2n) minimum energy ~ 8.13 MeV.
Note, lithium-7 has the worst (highest) activation energy. Strike #1!
Second, cross section values are not constant – they change, based on neutron energy. For a given energy, the ratio of the (n,2n) cross section to the total (mostly elastic scattering) cross section determines the “likelihood” of that neutron triggering an n,2n reaction during its encounter with the nucleus.
The table below applies to 14.1 MeV neutrons, the amount of energy a DT fusion neutrons has on its first encounter with nuclei inside the blanket. On subsequent encounters, the neutron has less energy, but, according to ENDF plots, the likelihood of an (n,2n) reaction remains relatively stable throughout the whole range where the reaction takes place.
Blanket [Cross_sections (barns)] Likelihood
Material (n,2n) elastic total (%)
7Li 0.032 1.0 1.4 2.3
9Be 0.5 1.0 1.5 33.
206Pb 2.2 2.8 5.3 42.
Note, lithium-7 has the lowest (over ten times worse) (n,2n) likelihood. Strike #2!
Third, the proportion of energy lost per collision determines the number of collisions that can occur before a neutron becomes too slow to trigger (n,2n) reactions. Most collisions are glancing deflections, but, to keep calculations manageable, only elastic head-on collisions were assumed. That assumption is inaccurate, but, importantly, if the goal is to allow back-of-the-envelope calculations and order-of-magnitude comparisons to be made between different blanket materials, then the relative influence of that assumption is inconsequential.
In elastic collisions, both momentum (mv) and kinetic energy (mvv/2) are conserved. When a neutron collides head-on with a nucleus, measured in A atomic mass units (amu), it will rebound with a velocity that is (A-1)/(A+1) of its initial velocity. Likewise, because kinetic energy is proportional to velocity squared, only ((A-1)/(A+1))^2 of its initial energy remains.
Blanket Mass [__Rebounding_neutron’s__] Energy (MeV) after Number of collisions
Material (amu) rel. Velocity rel. Energy 0 1 2 3 4 5
7Li 7 6/8 36/64 14.1 7.93 4.46 2.51 1.41 0.79
9Be 9 8/10 64/100 14.1 9.02 5.78 3.70 2.37 1.51
206Pb 206 205/207 0.9808 14.1 13.83 13.56 13.30 13.05 12.80
Note, after a single head-on collision with 7Li, a 14.1 MeV fusion neutron is left with only 7.93 MeV of energy, not enough to trigger an endothermic (8.30 MeV) 7Li(n,2n) reaction. In contrast, the same neutron could undergo five head-on collisions with 9Be before it lost the ability to multiply neutrons via 9Be(n,2n). And, whereas a single collision with 7Li only triggers the 7Li(n,2n) reaction 2.3% of the time, there is an 86% chance it would trigger 9Be(n,2n), because it could potentially undergo five collisions at 33% per collision. There is a 100% chance of 206Pb(n,2n), because of its 42% likelihood per collision and potential to undergo 28 collisions. Lithium-7 is, by far, the worst. Strike #3!
In summary, I could not find a single reason why anyone would consider using pure lithium for a fusion blanket. In addition, metallic lithium is flammable and it explodes on contact with water.
LikeLike
An open source version of MCNP, if you want it – https://docs.openmc.org/en/stable/
LikeLiked by 1 person
Thanks Nick, that is a very impressive looking program. I found documentation for a similar program, about 14 years ago, written by a U.S. government agency, but classified as a nuclear secret. I am surprised this program is opensource. MCNP programs require lots of time to development, over 100 man-years. I could have obtained the government’s version, but it cost over $500 and I needed to get official government clearance to use it. Well… some things are changing for the better. 🙂
LikeLike
Wow thanks, what a great detailed reply. I’m not really in a position to judge but all I can comment is having a 950 tonnes of beryllium sloshing around is scary. Wikipedia says it is a class 1 carcinogen, its toxicity is equivalent to Arsenic and Mercury and safe levels are of the order of micrograms per cubic meter. That would be a health and safety nightmare.
LikeLiked by 1 person
Thanks you for your reply. I was listening to the “What Fusion Needs: Solutions to Technical Challenges – Which areas do we need to progress? FUSION22” youtube presentation, and at 29:45 they start talking about the Lithium breeding blanket. The expert from UKAEA talked about the need for a Lithium 6 enrichment supply chain. Your blog suggests this is not needed as the naturally occuring Lithium 6/7 mix is quite adequate enough. I know you can’t answer for him, but can you understand why he may be stating this? Here is the link: https://www.youtube.com/watch?v=BTAS5L_9k1I
LikeLiked by 1 person
Well, the natural isotope balance is fine for us because we can capture 99% of the neutrons and make tritium from them. In a Tokamak, which is what the UKAEA does, this is not the case. Lithium 6 enrichment may indeed be needed. And the “breeding blanket” is an extremely difficult engineering challenge, whereas in our system, with flowing lithium inside the chamber, it is much easier. Producing “net tritium”, as he says, is a major issue for a Tokamak, one which we avoid by design
LikeLiked by 1 person
This all sounds very encouraging. With tritium currently at $2000 a gram and with high demand and low supply expected once all these new startup need to start tests, you could probably make as much, if not more, selling tritium from your new power plant as you could adding electricity to the grid.
LikeLike
I apologise that should be Tritium costs $30,000 a gram.
LikeLiked by 1 person
Nick, I enjoy reading everything you post. You should write a book. 🙂
Did you run TBR simulations of your reactor? I understand your approach uses a DT gas target embedded in some kind of plastic material. Did you take that material and the projectile into account? If you don’t intend to enrich your lithium, what do you intend to do when fusion depletes your circulating lithium of 6Li? Maybe you could sell it to FLiBe Energy. I understand they need 7Li for their molten salt thorium fission reactors. 🙂
LikeLiked by 1 person
Thanks very much! We have done TBR simulations and we do take the target into account. The target is quite a lot more complex than the simple plastic thing on the website. And it does have an effect, it downscatters the neutrons, not all but some. So they are not all at 14 MeV, there is a significant flux at a broad range of lower energies, although there is a peak at 14 MeV. The amount of lithium in the system and the amount converted to tritium mean that there is basically no depletion.
LikeLike
Nick, I mistakenly assumed you would know I was asking about a future commercial power plant using your approach to fusion. I was not asking about your current lab facility. So, to be clear, I’ll ask
again… What do you intend to do with the contents of your lithium blanket when, after years of operation, breeding tritium for a 1Gwe (2Gw thermal) commercial fusion power plant, it becomes depleted of its minor (<5%) isotope 6Li? Understand?
LikeLike
Hi Michael, I just double checked the maths for a 1 GWe plant with 50% thermal efficiency, as you’ve described. Producing this much power would mean fusing 150 kg of DT per year. I used an availability of 80%. This means 1.8e28 atoms of tritium, and to produce those atoms of tritium we need an equal number of lithium atoms, which is about 210 kg per year. For a 1 GWe plant a reasonable estimate for the total lithium inventory is 1000 tonnes. With 40 years of operation that means that the total mass of lithium converted is 0.84 % of the total. So the lithium is not meaningfully depleted.
LikeLiked by 1 person
Wow 1,000 tonnes per power station is a lot of lithium. The annual global production of Lithium is only 540,000 tonnes a year with increasing competition from battery manufacturers. I hope supply chain issues will not present as a limiting step to commercialisation. I hear they are mining lithium in Cornwall, maybe some of that supply can be reserved.
LikeLike
I guess what I read when I see your comment is, enough for 540 plants per year, which is a lot. I know that’s not a true reflection, as you say batteries. But there is enough for long term scaling
LikeLiked by 1 person
Thanks Nick, your math is correct, but I question your assumptions. Are you kidding me? 🙂 There is more lithium in your proposed 1000 tonne blanket than in a solid cube, higher than a three story building! Are you sure that’s enough? 😉 Also, when addressing my question about lithium-6 depletion, you answered “the total mass of lithium converted is 0.84%.” OK, that is correct, but it doesn’t answer my question. Breeding only consumes 6Li, not 7Li. If we start, more reasonably (ha ha ha), with a cube of lithium two stories high (7m x 7m x 7m), it would weigh 183 tonnes and initially contain 8883 kg of 6Li (4.85%). After 40 years of breeding tritium, it would have consumed 95% (8400 kg) and ceased to function (assuming that 0.2 percent lithium-6 is insufficient for breeding).
Am I making sense?
LikeLike
Nick, your wisdom is eternal. 🙂
In my previous reply, I disregarded one of your stipulations. You stated a 1000 tonne lithium blanket, and I wrongly introduced a 183 tonne blanket. Thus, I weakened your position by substituting a strawman. It is funny, and amazing that I did not realize it until later, that you nevertheless still won the argument, because the blanket was not depleted (still operational) right up until the end of the plant’s 40 year life.
LikeLike
Getting the same volume of isotopically pure Lithium6 would require access to 5% of the world wide annual production. So you are ahead over your rivals who need that. No wonder the guy from the UKAEA was worried about Lithium6 supply chains.
LikeLike